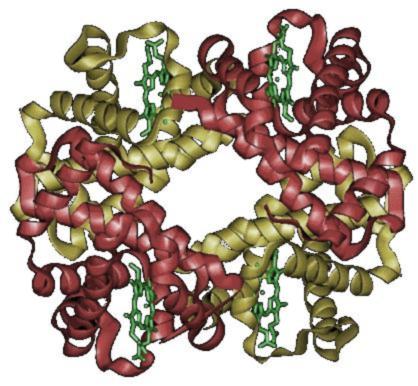
Folks, meet hemoglobin. Hemoglobin is a tetrameric protein. That means it's made out of 4 subunits. In this case, its subunits are called alpha, alpha, beta and beta. The two alphas and the two betas are identical.

Hemoglobin is reponsible for carrying the oxygen you breathe in to the cells throughout your body, via your bloodstream, so that your cells can carry out respiration and you can stay alive. How it accomplishes this task is.... wicked awesome.
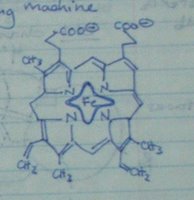 Here's another very important part of hemoglobin. It's called a "heme group", and although it's not a protein itself, one lies in the middle of each subunit. That means there are 4 heme groups per protein.
Here's another very important part of hemoglobin. It's called a "heme group", and although it's not a protein itself, one lies in the middle of each subunit. That means there are 4 heme groups per protein.The most special part about the heme group is the middle. There's a space there for 1 single iron atom to bind. You can see it in the picture. (Iron = Fe.) This iron atom is the critical, important part of the whole entire protein. You'll see why.
So, let's follow the path of an oxygen molecule. It gets breathed in, and it's hanging out in the alveoli, which are the little sacs at the very end of all the crazy trees that make up your lungs. Then hemoglobin comes swishing by in a capillary that goes by the alveoli. The oxygen molecule diffuses through the wall of the alveoli (don't worry right now about how that works), and it binds to the iron atom sitting in the middle of the heme group. That's the important part.
 Normally, iron atoms, unbound (Fe2+), have a certain size. They're pretty big. (If you want more details, it's because there are 4 unpaired electrons in the outer d orbitals of the atom. All of these atoms have the same spin, and that makes the atom paramagnetic.) What you need to know is that the iron atom is SO big, it doesn't quite fit in to the hole in the middle of the heme. It sticks out the top.
Normally, iron atoms, unbound (Fe2+), have a certain size. They're pretty big. (If you want more details, it's because there are 4 unpaired electrons in the outer d orbitals of the atom. All of these atoms have the same spin, and that makes the atom paramagnetic.) What you need to know is that the iron atom is SO big, it doesn't quite fit in to the hole in the middle of the heme. It sticks out the top. But when the iron atom binds the oxygen molecule you breathed in, it becomes smaller. (This is because the electrons reorganize in the d orbitals, and the spins now cancel each other out, and the atom becomes diamagnetic. Incidentally, this rearrangement from high energy orbitals to lower ones causes the red color of iron and blood.) Now, the iron atom fits in the hole in the middle of the heme *perfectly*.
Why does this matter? Because when the atom shrinks down, a particular part of the hemoglobin protein that sits next to it gets pulled down too. (It's Histadine 8 on the F chain, by the way.) Although it may not seem like pulling one small arm of a very large protein would make any difference it does!!
 In fact, it makes a very large difference. It causes the subunits of hemoglobin to change positions with respect to each other. The subunits kind of look like dumbells, and they rotate 15 degrees from a nice even X shape (like the crossbones on a pirate flag) to an uneven X. (This change in shape happens when 8 hydrogen bonds and some salt bridges are broken.)
In fact, it makes a very large difference. It causes the subunits of hemoglobin to change positions with respect to each other. The subunits kind of look like dumbells, and they rotate 15 degrees from a nice even X shape (like the crossbones on a pirate flag) to an uneven X. (This change in shape happens when 8 hydrogen bonds and some salt bridges are broken.)So the protein is a slightly different shape. Why do we care?
Now, this is the COOLEST part. The pressure of oxygen in your lungs is about 100 mmHg. The pressure of oxygen in your body tissues is about 30 mmHg. If you don't know about mmHg, it doesn't matter. All you need to know is that there's more oxygen in your lungs than in your tissues. Naturally, right? Remember, you just breathed in.
In the 100 mmHg areas in your lungs, it just so happens that the oxygen-bound/small-atom/uneven-X form of hemoglobin is quite stable. That's good! That means that when hemoglobin is around high concentrations of oxygen, it wants to bind it. (This is because molecules always end up in the most stable state possible.)
But hemoglobin is special, because it's sensitive to the difference between 100 mmHg and 30 mmHg. At 30 mmHg, the stability I described is no longer the case. In fact, it's more stable NOT being bound to oxygen. So the oxygen is going to fall off. This is absolutely essential. It means that when the hemoglobin shows up in your tissues in your body, where you have low oxygen pressure, the oxygen falls off. Hooray! Now your cells can use it.

No comments:
Post a Comment